BRAIN-COMPUTER INTERFACE & NEUROTECHNOLOGY
High-performance medical products for invasive and non-invasive use with the brain in research and clinical environments

JOIN THE SPRING SCHOOL 2024
140 hours of cutting-edge education over 10 days, featuring 85 speakers representing 86 institutions. Engage in 44 Hackathon projects led by 15 hosts, all focused on pushing the boundaries of neurotechnology.

WE ARE G.TEC
g.tec medical engineering produces invasive and non-invasive brain-computer interfaces (BCIs) and neurotechnology that are used worldwide to measure and analyze brain waves with the highest possible resolution. A key factor is the real-time data analysis which enables many new applications and experiments in neuroscience.

HAPPY CUSTOMERS
OUR NEWS

g.tec News #1 in 2024: Upcoming events and more news
140 HOURS OF CUTTING-EDGE EDUCATION 10 Days, 85 Speakers, 86 Institutions, 44 Hackathon projects Leave coffee and lunch breaks behind…

Open- and Closed-Loop Neuromodulation with MATLAB and Simulink
Neuromodulation in a nutshell Neuromodulation involves the deliberate alteration of nerve activity through targeted delivery of stimuli, such as electrical…

g.tec to Host World’s Premier BCI & Neurotechnology Summit With 2024 Spring School
As brain-computer interface (BCI) and neurotechnology continue to develop, there is growing interest in these emerging scientific areas, from both…

Mapping of the Central Sulcus using g.Pangolin Ultra High-Density EEG/EMG/ECG System
Understanding how the brain works is really important. One way to do this is by mapping the brain’s activity with…
BEHAVIORAL, COGNITIVE
NEUROSCIENCE
Use the complete hardware and software environment to study the human brain in real-time or offline, invasive or non-invasive, with or without electrical or magnetic stimulation of the brain or body.

COMPLETE SOLUTION
BRAIN-COMPUTER INTERFACE
g.tec’s rapid prototyping BCI environment allows you to realize many different applications with minimum effort. All major principles: slow waves, steady-state visual evoked potentials (SSVEP), motor imagery (MI) and evoked potentials (EP) are already implemented and can easily be used or modified.

HEALTHCARE
BIOMEDICAL ENGINEERING
g.tec’s BCI environment provides complete MATLAB-based research and development systems, including all hardware and software components needed for data acquisition, real-time and offline data analysis, data classification and neurofeedback. The environment enables you to quickly setup closed-loop experiments including non-invasive or invasive stimulation of brain and body.

NEUROPSYCHOLOGY
g.tec’s hardware and software is perfect for recording signals from different parts of the body in many different experiments. In combination with TMS (Transcranial Magnetic Stimulation) and tDCS (transcranial Direct-Current Stimulation) pulses, the impact of stimulation on the human body can be studied.

INVASIVE/NON-INVASIVE
BRAIN STIMULATION & MAPPING
Invasive and non-invasive stimulation and brain mapping techniques are used to determine brain functions in neuroscience, rehabilitation research and neurosurgery. g.tec’s electrical stimulators help you to understand cortical functions and perform brain-mapping experiments with a closed-loop system.
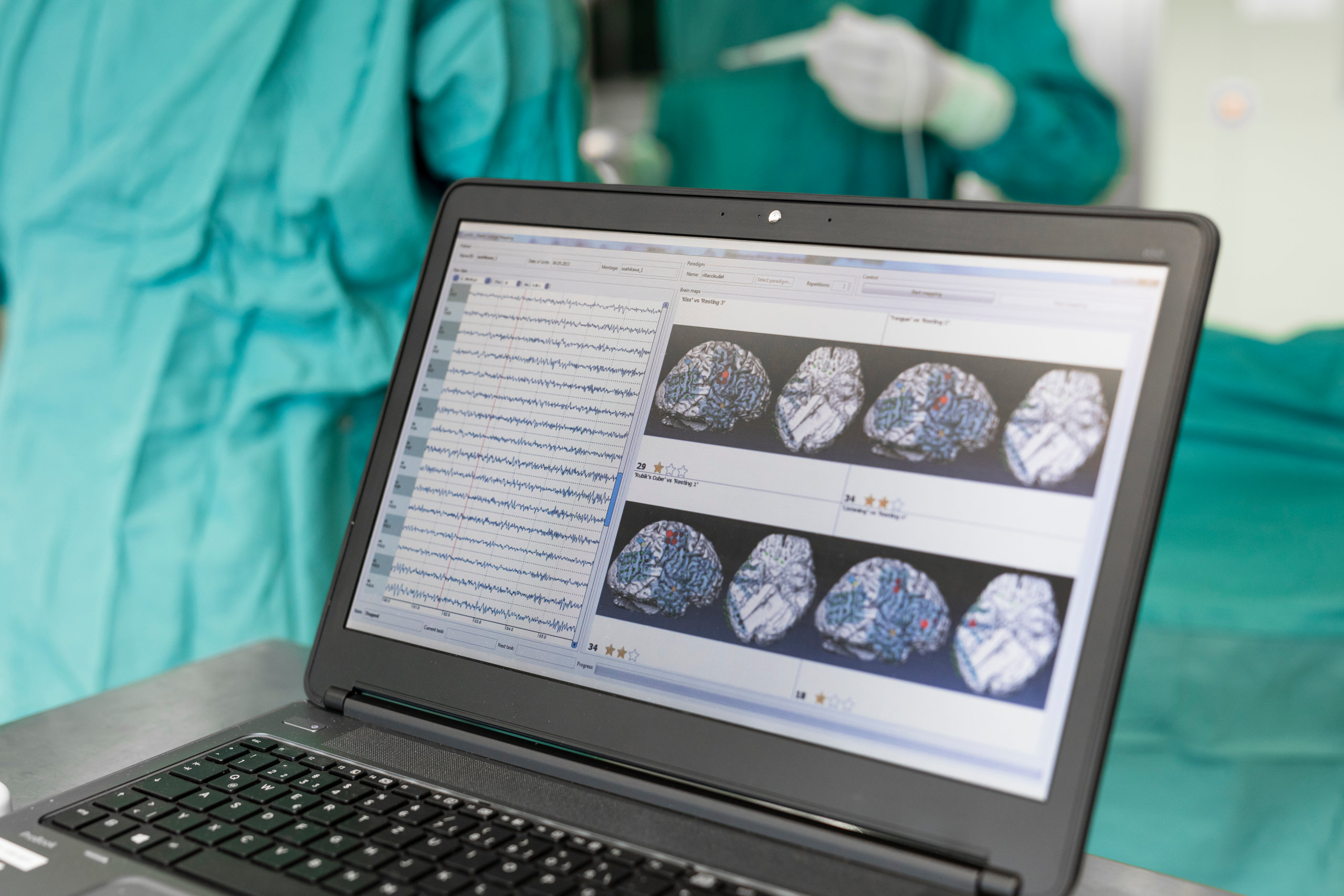
INVASIVE
NEUROMODULATION
Use g.tec’s rapid prototyping environment with up to 1000 channels to register neural spikes or ECoG and tune electrical stimulation of deep or cortical structures in real-time in order to develop treatments and therapy for Parkinson’s disease, psychiatric disorders like depression, pain and many more.
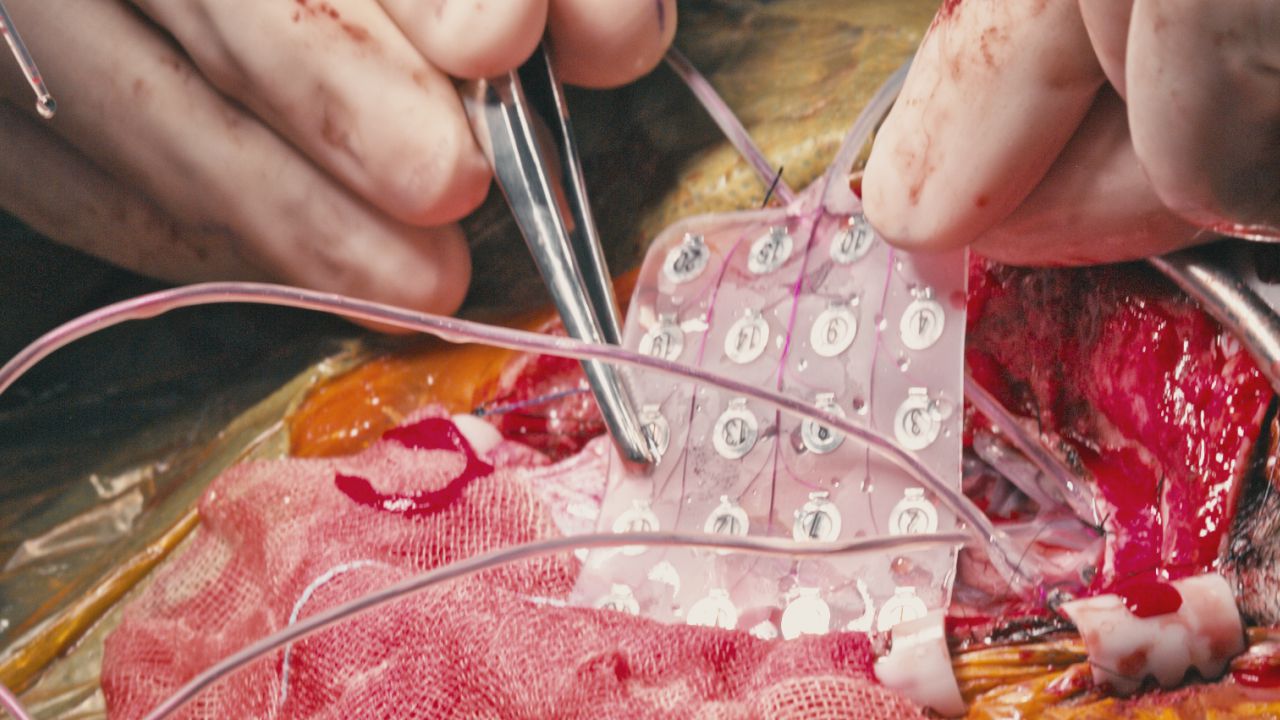
g.PANGOLIN HIGH-DENSITY EEG
g.Pangolin is the world’s first ultra-high density EEG electrode grid and biopotential amplifier system. The g.Pangolin EEG grids can be used with g.HIamp 256-channel biosignal amplifier to record high-resolution EEG signals. g.Pangolin EEG has 16 electrodes on a polygon shaped grid that can be mounted onto the head of a person. With the g.Pangolin, up to 1024 EEG channels can be recorded from the human head to get EEG data with never seen spatial resolution.

JOBS
EVENTS
-
From April, 22. 2024 to May, 01. 2024
BCI & NEUROTECHNOLOGY SPRING SCHOOL 2024